- Research article
- Open Access
- Published:
KbvR mutant ofKlebsiella pneumoniaeaffects the synthesis of type 1 fimbriae and provides protection to mice as a live attenuated vaccine
Veterinary Researchvolume53, Article number:97(2022)
Abstract
Klebsiella pneumoniaeis a leading cause of severe infections in humans and animals, and the emergence of multidrug-resistant strains highlights the need to develop effective vaccines for preventing such infections. Live attenuated vaccines are attractive vaccine candidates available in the veterinary field. We recently characterized that theK. pneumoniae kbvR(Klebsiellabiofilm and virulence regulator) mutant was a highly attenuated strain in the mice model. In the present study, the characterization, safety, and protective efficacy of ΔkbvRstrain as a live attenuated vaccine were evaluated. The synthesis and activity of type 1 fimbriae were increased in the ΔkbvRstrain. All mice inoculated by the subcutaneous route with 105, 106, and 107colony-forming units (CFU) doses of the ΔkbvRstrain survived. Subcutaneous immunization with two doses of 105or 107CFU ΔkbvRelicited a robust humoral immune response, and provided protection against the followingK. pneumoniaeintraperitoneal infection. The antisera of mice immunized with 105CFU dose improved the opsonophagocytic ability and complement-mediated lysis not only to the same serotype strain but also to the different serotype strain. The passive transfer of antisera from 105CFU dose-immunized mice provided protection againstK. pneumoniaeinfection. Overall, our results suggest the great potential of the ΔkbvRstrain as a novel vaccine candidate againstK. pneumoniaeinfections in herds or humans.
Introduction
Klebsiella pneumoniaeis a ubiquitous bacterium that can be found in a wide range of ecological niches including water, soil, vegetation, and insects [1,2,3]. It also can be recovered from the stools, nasopharyngeal swabs, or urine samples collected from companion and farm animals, such as dogs, cats, horses, cows, chickens, and pigs. To these animals, it is an essential cause of pneumonia, metritis, septicemia, and mastitis [4,5,6]. The infection leads to losses in milk production, food contamination, and high mortalities [7,8,9]. Meanwhile, theK. pneumoniaein animal hosts and along the food chain can be transmitted to humans and cause infection [10,11,12]. Treatment with antibiotics is an important method against bacterial pathogens. However, more than one-third ofK. pneumoniaeisolates in Europe are resistant to at least one class of the antimicrobial agents, and multidrug-resistant (MDR) strains have been recovered in clinical isolates not only from humans but also from companion animals and livestock [13,14,15].
Given the increasing multidrug resistance, new strategies are needed to control and prevent infection by MDRK. pneumoniaein animals and humans. Vaccination is regarded as another effective method to prevent infectious diseases. To date, there are no vaccines available againstK. pneumoniaeinfection and the development of vaccines againstK. pneumoniaehas become a priority in many countries. The schemes for vaccine development againstK. pneumoniaeinclude whole cell vaccines, ribosomal vaccines, outer membrane vesicles, and virulence factors including capsular polysaccharides (CPS), lipopolysaccharide, and protein-based vaccines [16,17,18,19,20,21]. Live attenuated vaccines are a mode of whole cell vaccines that are more immunogenic and can elicit long-lasting immune responses than the subunit vaccines, and they have a relatively low production cost. Although the problem of biological safety limits the use of live vaccines, they are suitable to immunizing large communities or herds. The main vaccines used for animals or humans against the infection ofBrucella melitensis,Yersinia pestis, andMycobacterium tuberculosisare still live attenuated vaccines [22,23,24,25,26]. ForK. pneumoniae, atonBdeletion mutant strain can induce a protective immune response against challenge with a wild-type strain [27].
KbvR is a regulator that has been found to reduce biofilm formation and the synthesis of CPS in theK. pneumoniaeNTUH-K2044 strain. The virulence of thekbvRmutant was significantly decreased in the murine model by intraperitoneal injection [28]. RNA-seq results show that the expression levels of type 1 fimbriae genes such asfimAandfimHwere increased inK. pneumoniae kbvRmutant strain [28]. Fimbriae are filamentous structures present on the cell surface of bacteria. The components of fimbriae have been shown to be promising vaccine candidates [29,30]. The selected T- and B-cell specific epitopes of type 1 fimbriae FimA, FimF, FimG, and FimH proteins have been estimated to be used as potential vaccine candidates againstK. pneumoniaeinfection, and six epitopes confirmed by molecular dynamics can be applied for vaccine development [31,32]. Thus, the ΔkbvRstrain might be a potentially viable option for the preparation of vaccines in preventingK. pneumoniaeinfections in humans and animals. In this study, the influence of KbvR on the synthesis of type I fimbriae was investigated. Then, experiments on the safety, antibody test, and protective efficacy of the ΔkbvRstrain in mice were performed to evaluate the potential of thekbvRmutant strain as a live attenuated vaccine.
Materials and methods
Bacterial strains and growth conditions
荚膜血清型K1 hypervirulentK. pneumoniaestrain NTUH-K2044 (WT), the NTUH-K2044kbvRgene deletion strain (ΔkbvR), and the capsular serotype K3 standardK. pneumoniae写明ATCC 13883被保存在我们的laborator应变y. All strains from frozen stocks were inoculated into Luria–Bertani (LB) broth and grown overnight with shaking at 37 °C. Then, the cultures were diluted 1:100 into fresh LB media the following day and cultured to the desired phase of growth.
RNA isolation and RT-PCR
Total RNA were extracted from mid-log phaseK. pneumoniaeWT and ΔkbvRstrains using an RNeasyMidi kit (Qiagen). Purified RNA was DNase-treated with RNase-free DNase I (Qiagen) to eliminate the residual DNA. The quality and quantity of RNA were determined via 1% agarose gel electrophoresis and with a Nano Drop 2000 UV–Vis Spectrophotometer (Thermo Scientific). The first-strand cDNA synthesis was conducted using the first-strand cDNA synthesis kit (Promega) and quantified. The relative expression levels offimAandfimHgenes in WT and ΔkbvRstrains were analyzed by agarose gel electrophoresis after amplifying cDNA by PCR. 16 S rRNA was used as the normalized gene. The relative mRNA levels were quantified by densitometry using ImageJ software and then calculated by dividing the densitometry offimA/fimHgene by the corresponding densitometry of 16 S rRNA.
Yeast agglutination assay
The presence and activity of type I fimbriae at the bacterial cell surface were assessed using the baker’s yeast suspended in phosphate-buffered saline (PBS) as previously described [33]. The mid-log phase of statically cultured WT and ΔkbvRstrains were washed twice and resuspended in PBS with 108colony-forming units (CFU)/mL. Equal cell number of 50 µL yeast cell suspension and 50 µL different bacterial suspensions were mixed on a glass slide. After shaking at room temperature for 3 min, the aggregation was assessed by visual inspection.
Transmission electron microscopy
The WT or ΔkbvRstrain was statically cultured in LB broth for 10 h and suspended in 1 mL of PBS for negative staining to observe the fimbriae directly [34]. A glow-discharged formvar-coated copper grid was floated on a drop of each bacterial suspension liquid for 3 min. Then, the excess liquid was drained off with filter paper, and the copper grid was stained with uranyl acetate for another 3 min followed by air-drying. The specimens were viewed under a transmission electron microscope (HT7800, Japan) operated at an accelerating voltage of 120 kV.
Mouse subcutaneous infections
Five- to six-week-old female BALB/c mice were obtained from HUNAN SJA laboratory animal Co., Ltd. to determine the virulence of ΔkbvRin mice by subcutaneous route. The NTUH-K2044 WT and ΔkbvRstrains were cultured to OD600 = 1.2 in LB broth and serially 10-fold diluted with sterile PBS. Appropriate dilutions were plated onto LB agar plates to calculate the numbers of CFU. A total of 10 mice for each group gathered from two experiments were injected subcutaneously with 105, 106CFU WT or 105, 106, 107CFU ΔkbvR. The mice were monitored daily for 14 days, and the survival rates were calculated to analyze the virulence of ΔkbvRby subcutaneous route.
Mouse immunizations and challenge experiments
Two different groups of BALB/c mice were respectively immunized with a low dose (105CFU) or high dose (107CFU) ΔkbvRstrain in 100 µL PBS two times for 4 weeks at 2-week intervals by subcutaneous route. The mice immunized with PBS were used as a normal control. All mice were monitored for skin reaction and weighed daily. At 28 days after the first immunization, the serum samples were collected from immunized (n = 10 for 105CFU dose from two experiments andn = 5 for 107CFU dose) and normal control (n = 10 from two experiments) mice to evaluate antibody production by enzyme-linked immunosorbent assay (ELISA). The liver, spleen, and lungs of the mice were processed, paraffin-embedded, microtome sectioned, and stained with hematoxylin and eosin to determine histopathological changes using an Olympus DP74 camera.
Two weeks after the second immunization, the mice (105CFU ΔkbvR-immunized group and PBS control group) were infected with 104or 105CFU log-phaseK. pneumoniaeWT strain via intraperitoneal route. At the 12 h post-infection, five immunized or control mice challenged by 105CFU WT strain were sacrificed. The blood was collected and plated onto LB plate to calculate the number of bacteria. Then, 3 mL LB medium was injected into the abdominal cavity of the mice. A total of 10 µL peritoneal lavage fluid was collected and serially diluted to calculate the bacterial number, while another 10 µL peritoneal lavage fluid was coated onto the slides and stained via the gram stain method to be observed by optical microscopy. Thereafter, the liver, spleen, and lungs were removed aseptically, weighed, and homogenized in PBS using a tissue homogenizer. The samples were serially diluted and spread on LB agar plate containing 100 µg/mL ampicillin to determine the bacterial counts. The remaining mice challenged by 104or 105CFU WT (n = 7 for each PBS control group andn = 8 for each immunized group) were observed every day for the next 14 days to evaluate the survival rate.
Enzyme-linked immunosorbent assay
Relative antibody levels in mice were determined by ELISA [35]. First, 96-well flat-bottom plates were coated with 100 µL lysate of whole NTUH-K2044 WT cells. This WT strain was grown in LB broth to OD600 = 1.2, collected by centrifugation, resuspended in PBS, and lysed by sonication. After overnight incubation at 4 °C, plates were washed three times with PBS including 0.05% Tween-20 (PBST), blocked with 5% nonfat dry milk for 1 h, and washed three times with PBST. The serum samples of immunized or normal control mice were diluted 1:400. The resulting titer was corrected at an optical density of 450 nm to about 0.1 of control serum, and 100 µL was applied per well followed by 1.5 h incubation at 37 °C. After washing with PBST, 100 µL of 1:3000 horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibodies was added and incubated at 37 °C for 1 h. The color was developed for 10 min in the dark by addition of 100 µL 3, 30, 5, 50-tetramethylbenzidine, and the reaction was stopped by adding 100 µL 2 M H2SO4. The optical density of the reaction was recorded at 450 nm using an ELISA plate reader and corrected by subtracting the optical density of a blank control.
Opsonophagocytic assay
For an opsonophagocytic assay, plasmid pLac-EGFP with a gene encoding green fluorescence protein was electroporated intoK. pneumoniaeNTUH-K2044 and ATCC 13,883 strains. The bacteria were cultured in LB at 37 °C to OD600 = 1.2, washed with PBS, and adjusted to approximately 107CFU/mL in RPMI 1640 medium. RAW264.7 macrophage cells were seeded in 24-well glass bottom culture plates and cultured in RPMI 1640 medium containing 2 mM glutamine and 10% (v/v) fetal calf serum [36]. For the opsonophagocytic assay, macrophages were infected with bacteria (NTUH-K2044 or ATCC 13,883) at a multiplicity of infection (MOI) of 50 bacteria/macrophage and 1 µL pooled immunized or normal mice sera (n = 5 for each group) in a 1100 µL reaction at 37 °C. After 120 min of contact, cells were washed twice with PBS and incubated for an additional 90 min with RPMI 1640 medium in the presence of 500 µg/mL gentamicin to eliminate the remaining extracellular bacteria. Then, the cells were washed, fixed, and stained with Alexa Fluor 594 dye (red) for actin cytoskeleton and DAPI (blue) for host cell nuclei. Finally, the cells were observed by confocal microscopy. For the opsonophagocytic activity against NTUH-K2044, the sum of the intracellular bacteria in the macrophages in different fields were calculated and quantified within 300 cells. For the opsonophagocytic activity against ATCC 13,883, the washed cells were lysed with 0.5% Triton X-100 and serial dilutions were plated on LB agar to quantify intracellular bacteria. The mean opsonophagocytic ratio of sera to ATCC 13,883 was determined using the formula = 100⋅CFU of intracellular phagocytized bacteria/CFU of input bacteria. Experiments were conducted on three independent occasions [36].
Complement-mediated antibacterial test
The complement-mediated antibacterial test was performed as previously described [36,37]. The pooled immunized or normal mice sera (n = 5) were heat-inactivated in a 56 ℃ water bath for 1 h. A total of 106bacterial cells (K. pneumoniaestrain NTUH-K2044 or ATCC 13,883) were co-incubated with 25% (v/v) human serum (providing the complement) donated by healthy volunteers with or without 5 µL heat-inactivated mice sera in a 50 µL reaction at 37 ℃ for 2 h. The colony count was calculated by the serial dilution method. The mean survival ratio was determined using the formula = 100⋅CFU of experimental wells with mice sera/CFU of control wells without mice sera.
Mouse passive immunization and challenge assay
For passive immunization experiments, the sera from 5 mice immunized with two doses of 105CFUK. pneumoniaeΔkbvRstrain (immunized sera) or from 5 mice injected with PBS (normal sera) were collected and pooled respectively. Then, pooled sera from immunized or normal mice were passively transferred to groups of 5 naïve female BALB/c mice in the dose of 1 µL respectively along with 100 µL 104CFU WT by intraperitoneal route. Mice were observed daily for 14 days, and survival was monitored [21].
Statistical analyses
The data were presented either as mean ± standard error (SD). A Mantel–Cox (log-rank) test was used to compare the survival curves, and the Studentt-test was used for the statistical comparisons between the two groups.
Results
Type 1 fimbriae biosynthesis is increased inkbvRmutant strain
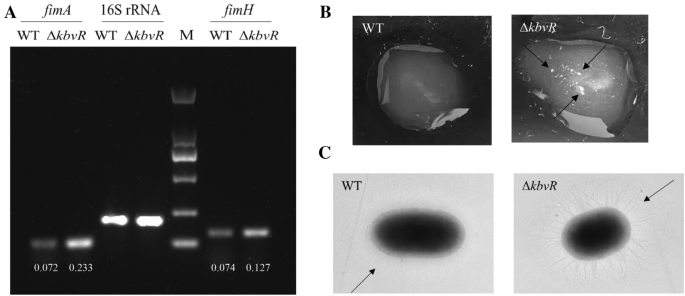
In our previous study, the RNA-seq results show that the transcription levels of gene cluster associated with type I fimbriae synthesis were increased in the ΔkbvRstrain (NCBI GenBank: No. SAMN17192750-SAMN17192755) [28]. The RT-PCR confirmed that the transcription levels offimAandfimH, the genes that encode the major subunit protein and the adhesion of type I fimbriae, respectively, were increased in thekbvRmutant strain. The relative mRNA levels offimAandfimHin ΔkbvR和WT 0.233和0.072和0.127和0。074, respectively (Figure1A). The yeast agglutination assay and transmission electron microscopy were used to directly observe the activity and production of type I fimbriae. As shown in Figure1B, the ΔkbvRstrain gave obvious clumps in a clear solution, whereas little agglutination was observed for WT at the same number of cells, indicating more type I fimbriae on the surface of ΔkbvRthan on that of WT, which mediated stronger yeast agglutination. The transmission electron microscopy images also show the existence of a large number of fimbriae on the surface of ΔkbvRcompared with that of WT (Figure1C). These results suggest that KbvR negatively regulated the synthesis and activity of type I fimbriae.
KbvR negatively regulates the expression of type I fimbriae. AmRNA levels offimAandfimHgenes were detected by RT-PCR. The overnight cultures of WT and ΔkbvRstrains were diluted 1:100 into fresh LB media and cultured to mid-log phase at 37 °C. Total RNA were extracted and RT-PCR was conducted with 16 S rRNA as the normalized gene. The relative mRNA levels offimAandfimHgenes were analyzed by agarose gel electrophoresis, quantified by densitometry using ImageJ software, and calculated by dividing the densitometry offimA/fimHgene by the corresponding densitometry of 16 S rRNA.BYeast agglutination assay. The mid-log phase WT and ΔkbvRcultures (108CFU/mL, 50 µL) were mixed with equal cell numbers of 50 µL yeast cell suspension and shaken at room temperature for 3 min. Large clumps (with arrows indicated) were observed in the ΔkbvRstrain compared with those in WT.C透射电子显微镜的图像K. pneumoniaeNTUH-K2044 and its ΔkbvRmutant, and arrows indicate the fimbriae.
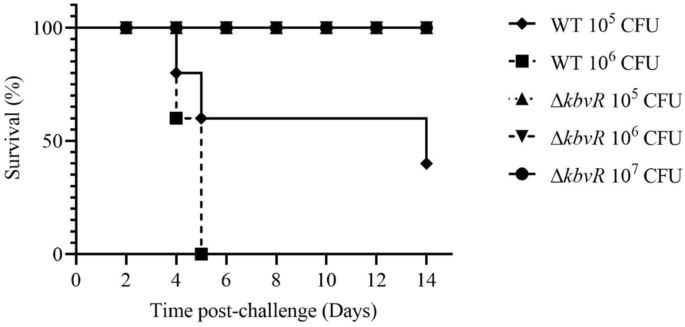
ΔkbvRstrain injected subcutaneously is avirulent in the mouse model
The 50% lethal dose (LD50) was equal to 5 × 102CFU for WT and more than 5 × 105CFU for ΔkbvRby intraperitoneal infection [28]. To assess the virulence of the ΔkbvRstrain infected by the subcutaneous route, the possible immunization route of live vaccine, the survival rate of mouse model infected subcutaneously by 105, 106CFU WT and 105, 106, 107CFUkbvRmutant strains were calculated for 14 days. The ΔkbvRstrain was also highly attenuated via the subcutaneous route. All mice infected by 106CFU WT died in 5 days, and the survival rate was decreased to 40% in the mice infected by 105CFU WT. On the contrary, no deaths occurred in the ΔkbvR-infected groups which were even challenged by the 107CFU dose (Figure2).
Subcutaneous immunization withkbvRstrain stimulates humoral immune response
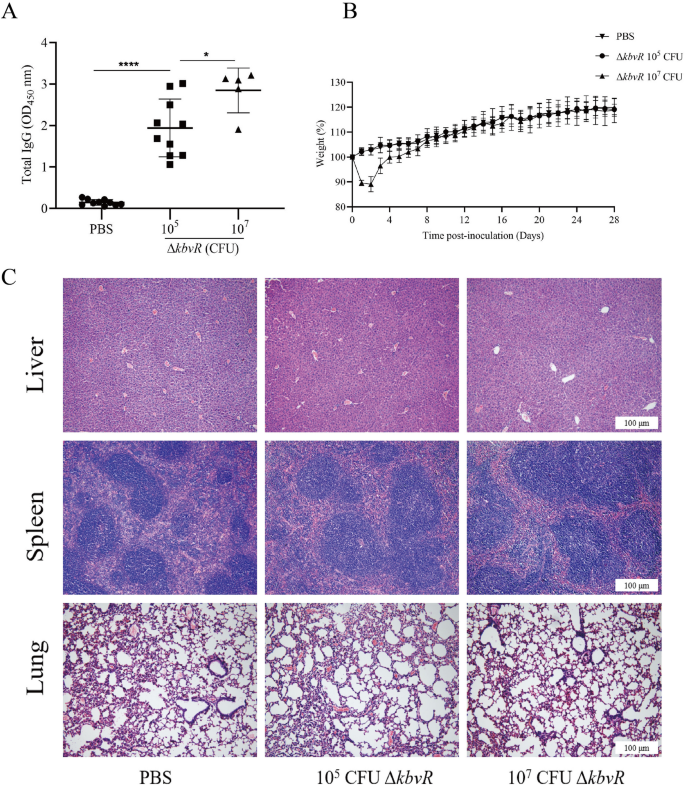
Given that ΔkbvRcould be a live attenuated vaccine candidate, the concentration of IgG in the mice immunized with two doses of 105or 107CFU ΔkbvRat 2-week intervals were quantified by ELISA assay. All immunized mice mounted IgG antibody response to wholeK. pneumoniaelysates at day 28 post-immunization, and the concentration was significantly higher than that of the PBS control group (P< 0.0001). The results indicate a positive effect of the ΔkbvRstrain on antibody induction. The 107CFU-immunized group had even higher antibody concentration than the 105CFU-immunized group (P< 0.05), suggesting that the increase in the immunized ΔkbvRdose might improve vaccine efficacy (Figure3A).
Immune responses to inoculation with 10CFU or 107CFU ΔkbvR. Mice were immunized with 105CFU (n = 10), 107CFU (n = 5) ΔkbvRor PBS (n = 10) by the subcutaneous route for two doses on days 0 and 14.ABlood was collected on day 28 post-inoculation, and the relative levels of serum IgG antibody against whole cell lysates of NTUH-K2044 were determined by ELISA. The optical density of the horseradish peroxidase reaction was recorded at 450 nm.BDaily weights of mice inoculated with 105CFU ΔkbvR, 107CFU ΔkbvR, or PBS over 28 days.CHistopathological examination of the liver, spleen, and lung of 105, 107ΔkbvRor PBS immunized mice on day 28 post-inoculation. The section was counterstained with hematoxylin and eosin. Scale bars = 100 μm. *:P< 0.05; ****:P< 0.0001.
The weights and the skin changes in the injection sites of mice throughout the immunization were measured as indicators of side effects. No skin changes at the sites of injection and weight loss were observed in all mice inoculated with 105CFU ΔkbvR. However, mice initially inoculated with 107CFU ΔkbvRhad significantly lower weights (about 12%) than PBS-inoculated controls from days 1–6 post the first inoculation (P< 0.05), and local adverse reactions such as skin lesion and a local hair loss at the injection site were observed (Figure3B). These differences were significant when analyzed by mixed ANOVA study. The mice recovered their body weight on day 8, and no significant weight differences from PBS control were observed after the second administration. The liver, spleen, and lungs of PBS control, 105, or 107CFU ΔkbvR-inoculated mice were examined histologically at 28 days after the first injection to further investigate the virulence and safety of ΔkbvRin mice. No histological lesions and inflammatory infiltrate were observed in any examined organ (Figure3C). These data demonstrate that, although the 107CFU-immunized dose induced higher humoral response, it also might have the local side effect. A dose of 105CFU ΔkbvRwhich was well within the range of safe and immunogenic doses was chosen for further evaluation.
ΔkbvRimmunization induces protection againstK.pneumoniaeintraperitoneal challenge
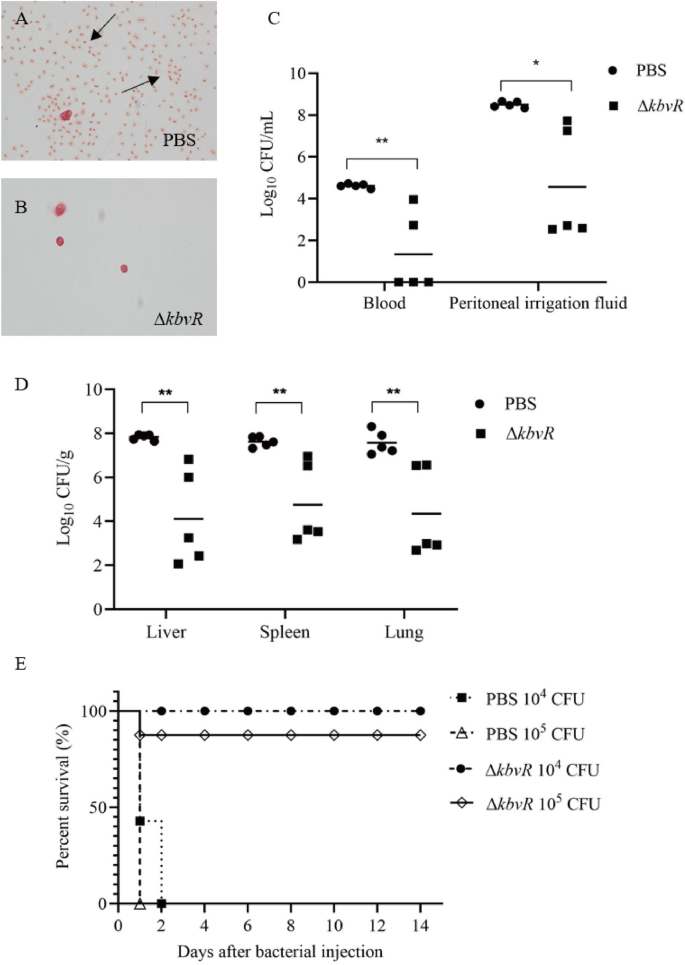
The attenuated virulence and stimulated antibody production ofkbvRmutant strain made it a candidate for consideration as a live attenuated vaccine. Thus, the ability of ΔkbvRstrain to confer protection against lethal intraperitoneal challenge with hypervirulentK. pneumoniaestrain NTUH-K2044 (WT, LD50 = 500 CFU) was tested. BALB/c mice were subcutaneously immunized with 105CFU ΔkbvRor PBS control at two doses, 14 days apart. A total of 2 weeks after the second vaccination, mice were intraperitoneally challenged with 104or 105CFU WT。在12 h后的挑战,5 imm的老鼠unized or normal control mice infected with 105CFU WT were sacrificed. The peritoneal washes were smeared, stained, and observed with a microscope. No bacteria were observed in the peritoneal lavage fluids of immunized mice. However, plenty ofK. pneumoniaewere found in the abdominal cavities of control mice (Figure4A and B). The blood, peritoneal washes, liver, lungs, and spleen were harvested to calculate the bacterial number. Immunized mice exhibited significant reductions in organ colonization in the peritoneal washes (1.86-log reduction), liver (1.9-log reduction), spleen (1.6-log reduction), lungs (1.74-log reduction), and blood (3.45-log reduction) compared with the bacterial number of PBS controls (Figure4C and D). Three of five immunized mice exhibited no detectable bacterial colonization in the blood. By 2 weeks post-challenge, none of the control mice survived beyond 48 h. By contrast, the immunized group had survival rates of 100% and 87.5% for 104CFU and 105CFU challenge, respectively (Figure4E). Overall, these results suggest that the immunization with 105CFU ΔkbvRconferred significant protection in mice againstK. pneumoniaechallenge.
ΔkbvRimmunization induces protection againstK. pneumoniaeintraperitoneal challenge.Two doses of 105CFU ΔkbvRstrain immunized mice (n = 21) or PBS (n = 19) inoculated normal mice were intraperitoneally infected with 104(n = 15) or 105(n = 25) CFU log-phase wild-type strain 2 weeks after the second immunization.A, BPeritoneal lavage fluids of immunized (n = 5) or normal mice (n = 5) were collected, stained, and observed with a microscope at 12 h after being infected with 105CFU WT. Arrows indicate the bacteria.C, DNumber of bacteria in peritoneal washes, liver, spleen, lung, and blood of immunized or normal mice were determined at 12 h after infection with 105CFU WT.ESurvival curves of immunized or normal mice challenged with 104or 105CFU WT (n = 7 for each PBS group andn = 8 for each ΔkbvRimmunized group) (P< 0.0001).
Antisera from immunized mice increase uptake ofK.pneumoniaeby macrophages in vitro
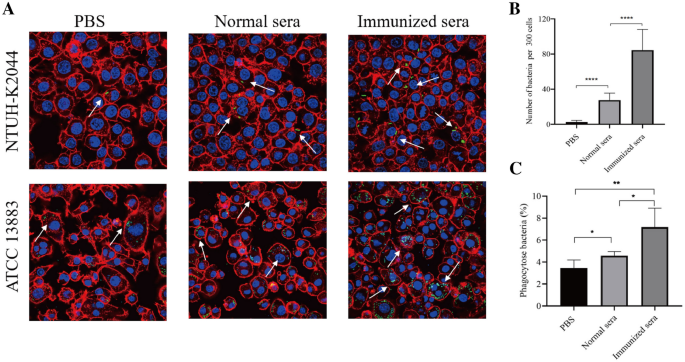
We measured the ability of the antibodies in immunized sera to promoteK. pneumoniaeuptake by opsonophagocytic assays for determining their functional activity. Phagocytic assay revealed that NTUH-K2044 was rarely presented inside macrophages without adding sera (Figure5A). Although the addition of normal sera from PBS control mice increased the phagocytic activity of macrophages, the pooled sera from ΔkbvRimmunized mice show statistically significant enhancement in opsonophagocytic activity compared with the pooled normal sera, whose anti-Klebsiellatiters were 1:3200 and 1:400 respectively. The average opsonophagocytized NTUH-K2044 were about 80 and 30 within 300 macrophages respectively (P< 0.0001) (Figure5B). The opsonophagocytic assay of the sera against theK. pneumoniaeserotype K3 strain ATCC 13,883 was also tested to evaluate whether the sera antibodies generated by immunization with the serotype K1 NTUH-K2044 ΔkbvRcross-reacted with the otherK. pneumoniaeserotype. The anti-phagocytosis ability of ATCC 13,883 against macrophage was much lower than that of NTUH-K2044 (Figure5A), which may be partly due to the difference in CPS. Meanwhile, the pooled sera from mice immunized with two doses of serotype K1 ΔkbvRstrain were also able to significantly increase macrophage uptake of ATCC 13,883 over the normal sera from PBS immunized animals (7.175% versus 4.575%,P< 0.05) (Figure5C). The antisera provided the opsonophagocytic activity not only to the K1 serotype but also to the K3 serotype strain, which implied that immunization with thekbvRgene deletion strain of K1 serotype might confer cross-protective immunity against other serotypes ofK. pneumoniae.
Determination of opsonophagocytic activity with immunized sera against NTUH-K2044 and ATCC 13,883 by confocal micrograph. ARAW264.7 macrophage cells were infected with the serotype K1 NTUH-K2044 or the serotype K3 ATCC 13,883 containing the plasmid pLac-EGFP (green) at an MOI of 50 with or without 1:1100 final dilutions of sera from PBS-inoculated normal mice or 105CFU ΔkbvR-immunized mice for 120 min, followed by treatment with 500 µg/mL gentamicin and staining with Alexa Fluor 594 dye (red) for actin cytoskeleton and DAPI (blue) for host cell nuclei. The opsonophagocytic activities of sera were observed under a confocal microscope. Arrows denote the intracellular bacteria. Scale bar = 5 μm.BThe opsonophagocytic activities of sera against NTUH-K2044 were counted under a confocal microscope. Data are presented as the intracellular bacterial numbers in 300 cells from three independent trials.CThe opsonophagocytic activities of sera against ATCC 13,883 were determined by the phagocytic rate. The mean opsonophagocytic ratio was calculated by the formula = 100⋅CFU of intracellular phagocytized bacteria/CFU of input bacteria. *:P< 0.05; **:P< 0.01; ****:P< 0.0001.
Antisera from immunized mice increase complement-mediated lysis ofK.pneumoniae
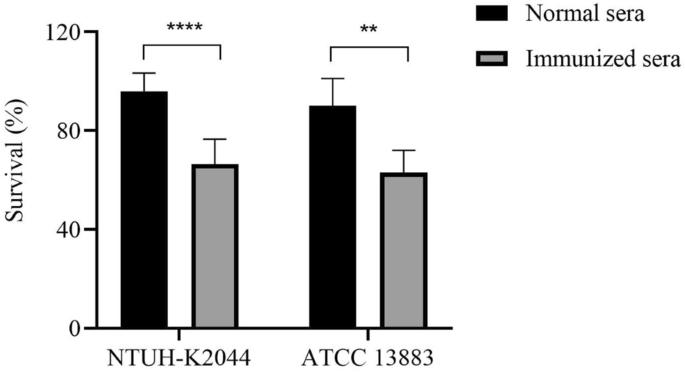
补体的激活是一个重要的组成部分the innate immunity of the antibacterial immunity. We characterized the complement activation by mice immunized sera in vitro to test whether antisera from immunized mice can increase complement-mediated lysis ofK. pneumoniae. TheK. pneumoniaeNTUH-K2044 or ATCC 13,883 strain was incubated with the heat-inactivated immunized or normal mice sera, along with human sera as a complement. Compared to the lysis induced by normal sera, the survival rate induced by the immunized sera against NTUH-K2044 and ATCC 13,883 were both significantly decreased (66.4% versus 95.9%; and 63% versus 90%) (Figure6). The above-mentioned results suggest strong complement activation after immunization with ΔkbvR, which might contribute to improving the antibacterial ability of the host immune system.
Immunized sera reduce survival rate of complement-mediated antibacterial test againstK. pneumoniae. TheK. pneumoniaestrain NTUH-K2044 or ATCC 13,883 (106CFU) were co-incubated with human complement with or without 10% heat-inactivated sera from pooled immunized or normal mice for 2 h at 37 °C. The survival ratio was determined using the formula = 100⋅CFU of experimental wells with mice sera/CFU of control wells without mice sera. **:P< 0.01; ****:P< 0.0001.
Passive transfer of antisera from immunized mice provides protection againstK.pneumoniaeinfection
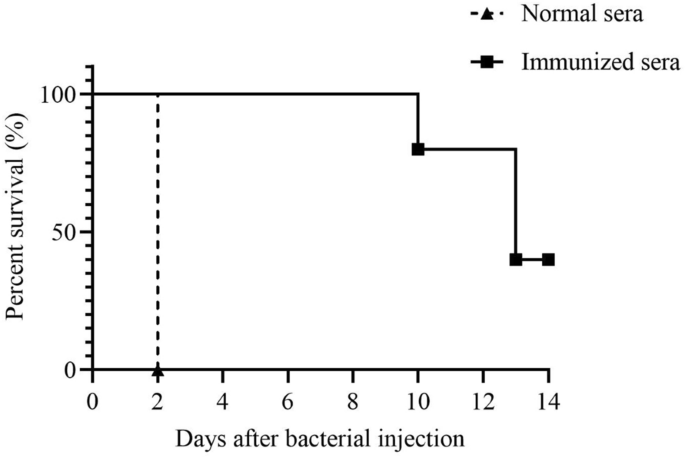
We determined whether passive transfer of immunized sera to naïve mice could provide protection against infection ofK. pneumoniaeto examine the protective role of antibodies in vivo. Pooled sera (n = 5) were obtained from BALB/c mice immunized with 105CFU ΔkbvR(immunized sera) or PBS control (normal sera). The 1 µL pooled immunized or normal sera were intraperitoneally injected into naïve mice (n = 5 per group) together with 100 µLK. pneumoniaeWT (approximately 104CFU)。所有的老鼠都是被动地转移到智慧h normal sera succumbed to infection within 2 days after injection. On the contrary, all mice treated with immunized sera resolved the infection for 8 days and 40% mice survived for 14 days (Figure7). These results support the idea that passive transfer of immunized sera is protective againstK. pneumoniaelethal infection in mice.
Discussion
Klebsiella pneumoniaeis an emerging zoonotic pathogen that causes various severe infections in humans and animals, and these infections become untreatable due to the emergence of MDR strains [11,38]. Therefore, vaccines againstK. pneumoniaethat prevent infection in veterinary practices and humans need to be developed urgently. Among the efforts, live attenuated vaccines are still an appealing approach for animals because of their high immunogenic potential, a greater number of different immunogens, and ability to elicit long-lasting protection. The basic requirements for live vaccines are to reduce the virulence and ensure they contain the highest number of antigenic determinants necessary for inducing protective immunity [39]. InK. pneumoniae, the CPS, fimbriae antigens (type I fimbriae and type III fimbriae), and outer membrane proteins (OmpK36 and OmpA) were proven to be potential antigenic determinants for the development of subunit vaccines [17]. Our previous study found that KbvR was associated with the virulence and the synthesis of CPS inK. pneumoniaeNTUH-K2044 [28]. The decreased expression of CPS in NTUH-K2044 ΔkbvRcontributes to the attenuation of strain, and the maintained CPS on the surface of ΔkbvRstrain should still stimulate the protective immune response against the infection of serotype K1 strain. Except for CPS, the synthesis of type I fimbriae was found to be negatively regulated by KbvR and the expression level of type I fimbriae was increased in the ΔkbvRstrain. Therefore, the weakened physical shielding effect of CPS on the fimbriae along with the reduction in CPS, and the increased synthesis of type I fimbriae on the surface, ultimately enhanced the activity and the chance of type I fimbriae to contact with the immunity system. The highly conserved fimbriae components have been generally shown to be promising candidates for inclusion in vaccines [40,41,42]. In addition, the structural proteins of type 1 fimbriae have been confirmed as effective protein carriers and immunogens in conjugates [43,44]. Overall, the maintained CPS and the increased synthesis of type 1 fimbriae made ΔkbvRstrain a promising live attenuated vaccine that can provide broad antigen exposure and serve as intrinsic adjuvant properties to prime durable immune responses.
Given that ΔkbvRcould be a live attenuated vaccine candidate, we chose to further characterize the safety and protective effect of this strain using the murine model by subcutaneous route, a used delivery method for live attenuated vaccine. The findings indicated a positive effect of ΔkbvRon antibody induction and that the immunization of the ΔkbvRstrain can stimulate the dose-dependent humoral immune response. The 107CFU dose-immunized group had higher antibody concentration than the 105CFU dose-immunized group. Although the mice immunized by 105CFU ΔkbvRstrain could provide 100% protection against the 104CFU WT strain by intraperitoneal challenge, one mouse died among 105CFU-challenged mice. When the higher dose 107CFU was used for immunization, 100% protection against 105CFU challenge at 14 days or even at 42 days after the second immunization were provided (data not shown). However, mice initially inoculated with 107CFU ΔkbvRstrain had significantly lower weights and had a local skin side effect. Therefore, the optimum immune dose of ΔkbvRwith the best protective effect and safety will be fully evaluated in the future.
Klebsiella pneumoniaeespecially K1/K2 serotype strains resist to phagocytosis and complement-mediated killing through the thick capsule at the cell surface [45]. The serum from patients with recurrentK. pneumoniaeliver abscess increased the opsonizing and killing efficacy, suggesting that the humoral immune response plays an important role in eliminating bacterial infection [46,47]. In this study, the opsonophagocytic assay and in vitro serum bactericidal activity demonstrated that the mice antisera from 105CFU ΔkbvR-immunized group prompted efficient phagocytosis by macrophages and induced complement-mediated lysis not only against the homologous (K1) serotype strain but also against the heterologous (K3) serotype strain. Thus, the broad immunogens of the live NTUH-K2044 ΔkbvRstrain might provide a cross humoral protection to other serotype strains. The passive transfer of antisera from ΔkbvRimmunized mice to naïve mice provided protection againstK. pneumoniaeinfection in vivo. The immunization with ΔkbvRstrain at 105CFU dose induced an effective antibody protection against infection.
In conclusion, we evaluated the safety and protective effect of KbvR regulator mutant strain. The results suggest that the ΔkbvRstrain can provide protection against the same serotype and different serotypes, which implies its potential as a live attenuated vaccine againstK. pneumoniaeinfection in animals or humans. In the future, the selection of an appropriate immunization dose of the ΔkbvRstrain which balances between attenuation and immunogenicity, and the cross-protective effect of ΔkbvRagainst additionalK. pneumoniaeserotype strains and MDR strains should be studied subsequently to provide a theoretical basis for developing a commercial vaccine.
References
Bagley ST (1985) Habitat association ofKlebsiellaspecies. Infect Control 6:52–58
Melo-Nascimento A, Treumann C, Neves C, Andrade E, Andrade AC, Edwards R, Dinsdale E, Bruce T (2018) Functional characterization of ligninolyticKlebsiellaspp. strains associated with soil and freshwater. Arch Microbiol 200:1267–1278
Poudel A, Hathcock T, Butaye P, Kang Y, Price S, Macklin K, Walz P, Cattley R, Kalalah A, Adekanmbi F, Wang C (2019) Multidrug-resistantEscherichia coli,Klebsiella pneumoniaeandStaphylococcusspp. in houseflies and blowflies from farms and their environmental settings. Int J Environ Res Public Health 16:3583
Ribeiro MG, de Morais ABC, Alves AC, Bolaños CAD, de Paula CL, Portilho FVR, de Nardi Júnior G, Lara GHB, de Souza Araújo Martins L, Moraes LS, Risseti RM, Guerra ST, Bello TS, Siqueira AK, Bertolini AB, Rodrigues CA, Paschoal NR, de Almeida BO, Listoni FJP, Sánchez LFG, Paes AC (2022)Klebsiella-induced infections in domestic species: a case-series study in 697 animals (1997–2019). Braz J Microbiol 53:455–464
Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE (2008) Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasiveKlebsiella pneumoniae–identification of the hypermucoviscosity phenotype. Vet Pathol 45:226–231
Williamson S (2017)Klebsiella pneumoniaesepticaemia in preweaned pigs. Vet Rec 180:443
Hertl JA, Schukken YH, Welcome FL, Tauer LW, Gröhn YT (2014) Pathogen-specific effects on milk yield in repeated clinical mastitis episodes inHolsteindairy cows. J Dairy Sci 97:1465–1480
Hu Y, Anes J, Devineau S, Fanning S (2021)Klebsiella pneumoniae: prevalence, reservoirs, antimicrobial resistance, pathogenicity, and infection: a hitherto unrecognized zoonotic bacterium. Foodborne Pathog Dis 18:63–84
Huynh BT, Passet V, Rakotondrasoa A, Diallo T, Kerleguer A, Hennart M, Lauzanne A, Herindrainy P, Seck A, Bercion R, Borand L, de la Pardos M, Delarocque-Astagneau E, Guillemot D, Vray M, Garin B, Collard JM, Rodrigues C, Brisse S (2020)Klebsiella pneumoniaecarriage in low-income countries: antimicrobial resistance, genomic diversity and risk factors. Gut Microbes 11:1287–1299
Rodrigues C, Hauser K, Cahill N, Ligowska-Marzęta M, Centorotola G, Cornacchia A, Garcia Fierro R, Haenni M, Nielsen EM, Piveteau P, Barbier E, Morris D, Pomilio F, Brisse S (2022) High prevalence ofKlebsiella pneumoniaein European food products: a multicentric study comparing culture and molecular detection methods. Microbiol Spectr 10:e0237621
Thorpe H, Booton R, Kallonen T, Gibbon MJ, Couto N, Passet V, Fernandez JSL, Rodrigues C, Matthews L, Mitchell S, Reeve R, David S, Merla C, Corbella M, Ferrari C, Comandatore F, Marone P, Brisse S, Sassera D, Corander J, Feil EJ (2021) One Health or Three? Transmission modelling ofKlebsiellaisolates reveals ecological barriers to transmission between humans, animals and the environment. BioRxiv 8:455249
Wareth G, Neubauer H (2021) The Animal-foods-environment interface ofKlebsiella pneumoniaein Germany: an observational study on pathogenicity, resistance development and the current situation. Vet Res 52:16
Carvalho I, Alonso CA, Silva V, Pimenta P, Cunha R, Martins C, Igrejas G, Torres C, Poeta P (2020) Extended-spectrum beta-lactamase-producingKlebsiella pneumoniaeisolated from healthy and sick dogs in Portugal. Microb Drug Resist 26:709–715
Guerra B, Fischer J, Helmuth R (2014) An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol 171:290–297
Harada K, Shimizu T, Mukai Y, Kuwajima K, Sato T, Usui M, Tamura Y, Kimura Y, Miyamoto T, Tsuyuki Y, Ohki A, Kataoka Y (2016) Phenotypic and molecular characterization of antimicrobial resistance inKlebsiellaspp. isolates from companion animals in Japan: clonal dissemination of multidrug-resistant extended-spectrum β-lactamase-producingKlebsiella pneumoniae. Front Microbiol 7:1021
Ahmad TA, El-Sayed LH, Haroun M, El Hussein AA, El Ashry SH (2012) Development of immunization trials againstKlebsiella pneumoniae. Vaccine. 30:2411–2420
Assoni L, Girardello R, Converso TR, Darrieux M (2021) Current stage in the development ofKlebsiella pneumoniaevaccines. Infect Dis Ther 10:2157–2175
Babu L, Uppalapati SR, Sripathy MH, Reddy PN (2017) Evaluation of recombinant multi-epitope outer membrane protein-basedKlebsiella pneumoniaesubunit vaccine in mouse model. Front Microbiol 8:1805
Gorden PJ, Kleinhenz MD, Ydstie JA, Brick TA, Slinden LM, Peterson MP, Straub DE, Burkhardt DT (2018) Efficacy of vaccination with aKlebsiella pneumoniaesiderophore receptor protein vaccine for reduction ofKlebsiella mastitisin lactating cattle. J Dairy Sci 101:10398–10408
Hussein KE, Bahey-El-Din M, Sheweita SA (2018) Immunization with the outer membrane proteins OmpK17 and OmpK36 elicits protection againstKlebsiella pneumoniaein the murine infection model. Microb Pathog 119:12–18
Lee WH, Choi HI, Hong SW, Kim KS, Gho YS, Jeon SG (2015) Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med 47:e183
Achkar JM (2019) Prospects and challenges of a new livetuberculosisvaccine. Lancet Respir Med 7:723–725
Demeure CE, Derbise A, Guillas C, Gerke C, Cauchemez S, Carniel E, Pizarro-Cerdá J (2019) Humoral and cellular immune correlates of protection against bubonic plague by a liveYersinia pseudotuberculosisvaccine. Vaccine 37:123–129
Derbise A, Guillas C, Gerke C, Carniel E, Pizarro-Cerdà J, Demeure CE (2020) Subcutaneous vaccination with a live attenuatedYersinia pseudotuberculosisplague vaccine. Vaccine 38:1888–1892
Lalvani A, Sridhar S (2010) BCG vaccination: 90 years on and still so much to learn. Thorax 65:1036–1038
Pandey A, Cabello A, Akoolo L, Rice-Ficht A, Arenas-Gamboa A, McMurray D, Ficht TA, de Figueiredo P (2016) The case for live attenuated vaccines against the neglected zoonotic diseasesBrucellosisandBovine Tuberculosis. PLoS Negl Trop Dis 10:e0004572
Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT (2008) Serum-induced iron-acquisition systems and TonB contribute to virulence inKlebsiella pneumoniaecausing primary pyogenic liver abscess. J Infect Dis 197:1717–1727
Xu L, Wang M, Yuan J, Wang H, Li M, Zhang F, Tian Y, Yang J, Wang J, Li B (2021) The KbvR regulator contributes to capsule production, outer membrane protein biosynthesis, antiphagocytosis, and virulence inKlebsiella pneumoniae. Infect Immun 89:e00016–21
Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S (2013) Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 31:1210–1216
Ramezanalizadeh F, Owlia P, Rasooli I (2020) Type I pili, CsuA/B and FimA induce a protective immune response againstAcinetobacter baumannii. Vaccine 38:5436–5446
Rostamian M, Farasat A, Chegene Lorestani R, Nemati Zargaran F, Ghadiri K, Akya A (2022) Immunoinformatics and molecular dynamics studies to predict T-cell-specific epitopes of fourKlebsiella pneumoniaefimbriae antigens. J Biomol Struct Dyn 40:166–176
Zargaran FN, Akya A, Rezaeian S, Ghadiri K, Lorestani RC, Madanchi H, Safaei S, Rostamian M (2021) B cell epitopes of four fimbriae antigens ofKlebsiella pneumoniae: a comprehensive in silico study for vaccine development. Int J Pept Res Ther 27:875–886
Blumer C, Kleefeld A, Lehnen D, Heintz M, Dobrindt U, Nagy G, Michaelis K, Emödy L, Polen T, Rachel R, Wendisch VF, Unden G (2005) Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA ofEscherichia coli. Microbiology 151:3287–3298
Kim KW, Jung WK, Park YH (2008) Comparison of pre-stain suspension liquids in the contrasting ability of neutralized potassium phosphotungstate for negative staining of bacteria. J Microbiol Biotechnol 18:1762–1767
Zhao X, Zeng X, Dai Q, Hou Y, Zhu D, Wang M, Jia R, Chen S, Liu M, Yang Q, Wu Y, Zhang S, Huang J, Ou X, Mao S, Gao Q, Zhang L, Liu Y, Yu Y, Cheng A (2021) Immunogenicity and protection efficacy of aSalmonella entericaserovar Typhimuriumfnr,arcAandfliCmutant. Vaccine. 39:588–595
Kobayashi SD, Porter AR, Freedman B, Pandey R, Chen L, Kreiswirth BN, DeLeo FR (2018) Antibody-mediated killing of carbapenem-resistant ST258Klebsiella pneumoniaeby human neutrophils. mBio 9:e00297–e00218
Hu G, Chen X, Chu W, Ma Z, Miao Y, Luo X, Fu Y (2022) Immunogenic characteristics of the outer membrane phosphoporin as a vaccine candidate againstKlebsiella pneumoniae. Vet Res 53:5
Cheng F, Li Z, Lan S, Liu W, Li X, Zhou Z, Song Z, Wu J, Zhang M, Shan W (2018) Characterization ofKlebsiella pneumoniaeassociated with cattle infections in southwest China using multi-locus sequence typing (MLST), antibiotic resistance and virulence-associated gene profile analysis. Braz J Microbiol 49:93–100
Frey J (2007) Biological safety concepts of genetically modified live bacterial vaccines. Vaccine 25:5598–5605
Fan Q, Sims T, Sojar H, Genco R, Page RC (2001) Fimbriae ofPorphyromonas gingivalisinduce opsonic antibodies that significantly enhance phagocytosis and killing by human polymorphonuclear leukocytes. Oral Microbiol Immunol 16:144–152
Starks CM, Miller MM, Broglie PM, Cubbison J, Martin SM, Eldridge GR (2021) Optimization and qualification of an assay that demonstrates that a FimH vaccine induces functional antibody responses in women with histories of urinary tract infections. Hum Vaccin Immunother 17:283–292
Thankavel K, Madison B, Ikeda T, Malaviya R, Shah AH, Arumugam PM, Abraham SN (1997) Localization of a domain in the FimH adhesin ofEscherichia colitype 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest 100:1123–1136
Liu ZF, Chen JL, Li WY, Fan MW, Li YH (2019) FimH as a mucosal adjuvant enhances persistent antibody response and protective efficacy of the anti-caries vaccine. Arch Oral Biol 101:122–129
Witkowska D, Mieszała M, Gamian A, Staniszewska M, Czarny A, Przondo-Mordarska A, Jaquinod M, Forest E (2005) Major structural proteins of type 1 and type 3Klebsiella fimbriaeare effective protein carriers and immunogens in conjugates as revealed from their immunochemical characterization. FEMS Immunol Med Microbiol 45:221–230
Li B, Zhao Y, Liu C, Chen Z, Zhou D (2014) Molecular pathogenesis ofKlebsiella pneumoniae. Future Microbiol 9:1071–1081
Banerjee K, Motley MP, Diago-Navarro E, Fries BC (2021) Serum antibody responses against carbapenem-resistantKlebsiella pneumoniaein infected patients. mSphere 6:e1335–e1320
Yeh FC, Yeh KM, Siu LK, Fung CP, Yang YS, Lin JC, Chang FY (2012) Increasing opsonizing and killing effect of serum from patients with recurrent K1Klebsiella pneumoniaeliver abscess. J Microbiol Immunol Infect 45:141–146
Acknowledgements
The authors want to thank Enpapers (Jinan, China) who provided professional writing services, and our colleagues who provided technical and practical assistance.
Funding
This work was funded by Hubei Province’s Outstanding Medical Academic Leader Program, the National Natural Science Foundation of China (81902034), the Innovative Research Program for Graduates of Hubei University of Medicine (YC2022002), the Advantages Discipline Group (Public health) Project in Higher Education of Hubei Province (2021–2025) and the Cultivating Project for Young Scholar at Hubei University of Medicine (2021QDJZR021).
Author information
Authors and Affiliations
Contributions
BL designed the research project and wrote the manuscript. FZ and YM performed all major experiments. LX and YT participated in feeding and dissecting the mice. HL and ML completed the microscopy scanning. JX and RM participated in cell culture. ML performed the data analyses. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
批准的所有动物实验的动物Care and Use Committee of Hubei University of Medicine (Reference Number: HBMU 2021 − 108) and complied with all ethical and husbandry regulations. The use of human serum was approved by the Institutional Review Board of Hubei University of Medicine and Dongfeng General Hospital affiliated to Hubei University of Medicine (Reference Number: 2021-TH-107).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/)适用于汽车列车的数据可用le, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, F., Meng, Y., Xu, L.et al.KbvR mutant ofKlebsiella pneumoniaeaffects the synthesis of type 1 fimbriae and provides protection to mice as a live attenuated vaccine.Vet Res53, 97 (2022). https://doi.org/10.1186/s13567-022-01116-y
Received:
Accepted:
Published:
DOI:https://doi.org/10.1186/s13567-022-01116-y
Keywords
- Klebsiella pneumoniae
- KbvR
- vaccine
- immune response